English

行业
加入成千上万的行业领导者的行列,与 Chiggo 一起推动产品创新。

解决方案
从原材料到生产和产品增强的一站式解决方案。

资源
您需要了解的有关数字化制造的一切。
我们每天都会遇到尼龙,它首先用作织物的丝绸替代品,在第二次世界大战期间,它出现在降落伞,生命式绳索,甚至是防弹背心衬里。今天,尼龙是最受欢迎的工程塑料,由于其高强度比率,自润滑耐磨性,化学和热稳定性以及加工多功能性。

尼龙是一个合成聚合物家族的商品名称,称为聚酰胺,最初是由杜邦(Dupont)在1935年至1937年间首次开发的。其分子链由重复–NH – CO-(酰胺)链接组成,这些链之间的氢键产生了增加的结晶度。这种结构使尼龙具有高熔点,出色的耐化学性能和优质的电绝缘特性。作为热塑性塑料,可以将尼龙纺成纤维,铸成膜或注射成复杂的形状,并可以用添加剂修饰以实现广泛的特性。在接下来的部分中,我们将探讨几个最常见的尼龙等级,以及它们独特的特性如何适合不同的应用。
在深入了解细节之前,下表提供了每个尼龙等级的关键特征的简洁概述。
| 尼龙等级 | 单体使用 | 化学结构(重复单位) | - (ch₂) - 计数 | 拉伸强度(MPA) | 休息时伸长(%) | 弯曲模量(GPA) | 冲击阻力 | 吸收水分 | 融化温度。 (°C) | 耐化学性 | 维稳定性 |
| PA6 | ε-caprolactam | - [NH - (CH₂)₅– co]n | 5 | 80–90 | 50–300 | 〜2.5 | 高(非常艰难) | 〜2.8(饱和时最多〜9) | 〜220 | 非常好;被强酸/碱攻击 | 公平(湿度膨胀) |
| PA6/6 | 六甲基二胺 +脂肪酸 | - [NH - (CH₂)₆– NH – Co–(Ch₂)₄– co]n | 6,4 | 85–95 | 20–80 | 〜3.0 | 中等(更脆) | 〜2.5(饱和时最多〜8) | 255–265 | 优秀的油/燃料耐药性;气体渗透率低 | 公平(湿度膨胀) |
| PA4/6 | 1,4-二氨基丁烷 +脂肪酸 | - [NH - (CH₂)₄– NH – Co–(Ch₂)₄– co]n | 4,4 | 90–100 | 〜50 | 〜3.2 | 高(非常艰难) | 〜3.8(高于PA6/6) | 〜295 | 非常好;类似于PA6/6(抵抗燃料/油) | 公平 - 贫困(吸收最多的水分) |
| PA11 | 11-氨基酸酸 | - [NH - (CH₂)₁₀– co]n | 10 | 50–60 | 200–300 | 〜0.9 | 中等(灵活) | 〜0.25(饱和时最多〜2.5) | 〜188 | 出色的;出色的碳氢化合物和耐化学性 | 优秀(最小肿胀) |
| PA12 | 月la龙(或HMDA +十二烷酸) | - [NH - (CH₂)₁₁– co]n | 11 | 50–70 | 200–300 | 〜1.4 | mod – High(非常延性) | 〜0.25(饱和时最多〜1–2) | 〜178 | 出色的;非常耐燃料,溶剂,天气 | 优秀(最尺寸稳定) |
| PA6/10 | 六甲基二胺 +皮脂酸 | - [NH - (CH₂)₆– NH – Co–(Ch₂)₈– co]n | 6,8 | 60–70 | 〜150 | 〜2.1 | 高(寒冷的坚韧) | 〜1.5(低) | 220–225 | 优秀的化学和盐耐药性 | 好(水分吸收低) |
| PA6/12 | 六甲基二胺 +十二烷酸酸 | - [NH - (CH₂)₆– NH – Co–(Ch₂)₁₀– co]n | 6,10 | 60–65 | 〜200 | 〜2.2 | mod – High(硬) | 〜0.25(非常低) | 215–218 | 出色的;对燃料非常抗性,油 | 优秀(湿度高度稳定) |
笔记
拉伸和伸长值是针对未增强的尼龙(近似范围)。在〜50%的相对湿度(近似)下,在平衡下给出水分吸收,大多数尼龙的全水饱和值更高。 “冲击力”是指缺口撞击(IZOD/Charpy)。所有尼龙对油,油脂和碳氢化合物均具有良好的耐化学性。仅在显着的地方注意差异。
尼龙名称中的数字告诉您其分子构建块。单个数字(例如,尼龙6、11或12)来自乳糖或氨基酸的开环聚合,其中数量等于单体中的碳原子。两个数字(例如,尼龙6/6、6/12、4/6或6/10)是指二胺(第一个数字=其碳计数)和二氨酸(第二个数字=其碳计数)之间的冷凝反应。
平均–CH₂–段长度(n)控制酰胺链接之间的间距和–NH··O = C-氢键的数量,可以形成每单位长度。较大的n表示较长的亚甲基段,从而降低氢键密度并通常会降低结晶度。例如,PA12(n = 11)具有最长的间距和最低的结晶度,而PA4/6(n =(4 + 4)/2 = 4)的段最短,最高的氢键密度和最大的结晶度。如果您引入芳香环,共聚物块,填充剂或其他专业修饰符,这些结构更改会破坏规律性和转移结晶度,因此始终请参考特定的数据表或测试数据以了解其效果。
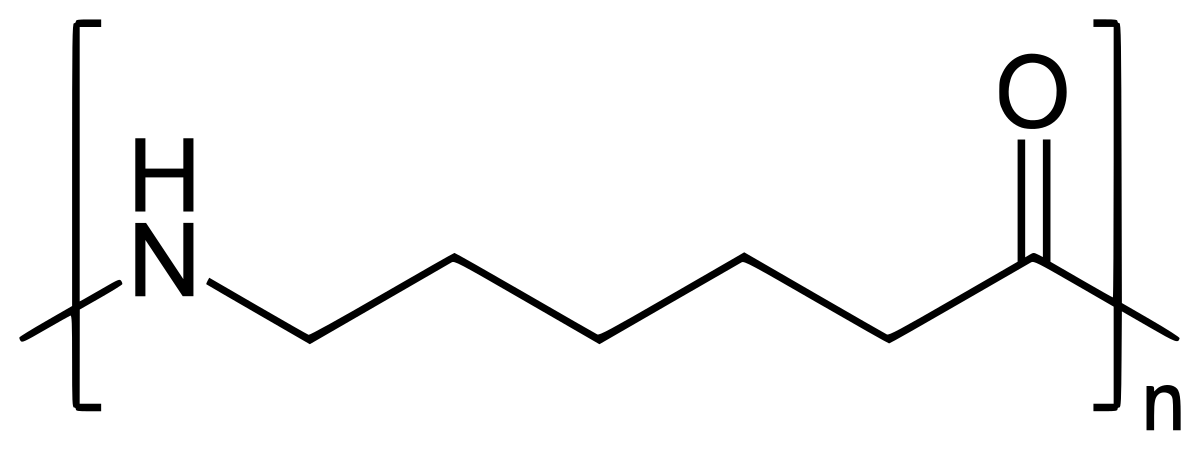
尼龙6(PA6)是一种半晶聚酰胺,该聚酰胺是通过ε-丙二酰酰胺分子的环式聚合产生的。它出色的功能之一是抗冲击力的出色。即使在低温下也不会破裂,它也会吸收冲击。 PA6还具有高抗拉力,自润滑性能和出色的耐磨性。结果,PA6是通用工程组件的首选,需要平衡强度,耐磨性和韧性,例如齿轮,轴承衬套和汽车进气歧管。在光纤部门,它被广泛用于地毯,纺织线和轮胎线中。与PA6/6和长链尼龙(如Pa11和Pa12)相比,PA6较大的熔点约为220°C,并且更逐渐结晶,PA6更易于处理,可提供较低的模具收缩和更平滑的饰面。这种易于成型使PA6特别适合于体育场座椅和枪支框架等复杂或薄壁的零件。
PA6在常见尼龙中具有最高的水分吸收,因此它可能不是暴露于湿度变化的精确零件的理想选择。对于紧密耐受性的应用,建议使用密封或预直联。
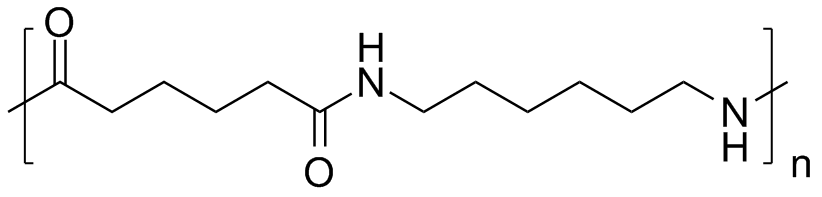
尼龙6/6(PA66)是原始的尼龙之一,在许多方面与尼龙6非常相似,但是它具有更高的结晶聚合物链。结果,它具有比尼龙6更高的拉伸强度和刚度。它也更难,更耐磨损,从而使高载荷或高摩擦应用有益于耐磨性。尼龙6/6的熔点约为260°C(500°F),比尼龙6高 - 因此,它可以在软化之前承受更高的工作温度,并且适用于更苛刻的热环境。权衡是加工性的:尼龙6/6难以塑造或挤压,需要更高的熔体和霉菌温度,并且倾向于与尼龙6相比显示出更大的霉菌收缩。
尼龙6/6也比尼龙6少于吸收水分,但仍然是吸湿性的,因此必须考虑耐受耐受性的部件的湿度。通常,它比尼龙6较小。换句话说,尼龙6更适合撞击强度或振动阻力,而当较高的屈服强度,刚度和耐热性最重要时,尼龙6/6是首选的。实际上,当需要额外的性能时,尼龙6/6通常用于与尼龙6的类似应用中,例如,高强度的机械零件,齿轮,外壳和汽车内部塑料组件,这些组件会看到升高的温度。它在工业机械,工具和电气组件中也很常见,它在宽温度范围内保持强度并提供良好的介电特性。
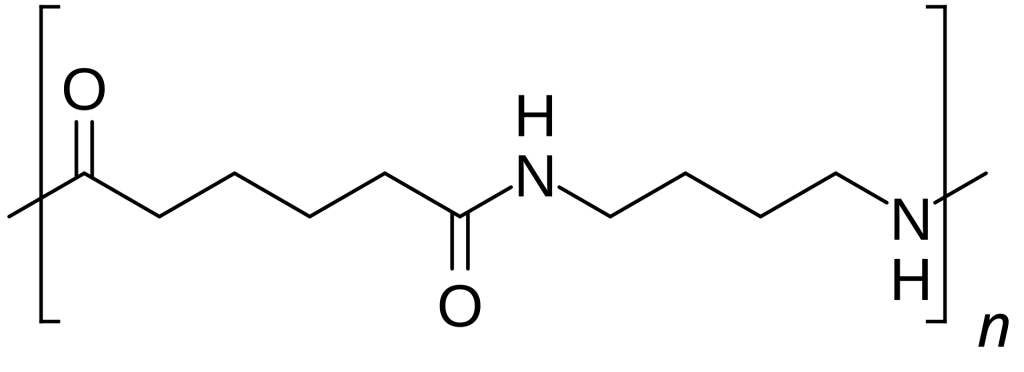
作为另一个短链脂肪族尼龙,PA4/6最与机械和热曲线中的PA66匹配。该聚合物具有高度结晶的结构 - 比PA6或PA66更重要,这是二胺的对称性和短长度。结果,PA4/6具有更高的熔点和较高的拉伸强度。在脂肪族尼龙中,在进入更专业的聚合物家族之前,它实际上是在机械性能的顶部附近。它还可以更快地结晶,从而实现较短的成型周期和潜在的较高的疲劳性耐药性。 PA4/6的冲击韧性可以超过PA66(尤其是在缺口测试中),这是值得注意的。
不利的一面是,PA4/6比PA66吸收更多的水分,并且生产(和购买)的成本更高。有人可能会说PA4/6以损失尼龙的性能提高了栏杆,而牺牲了水分稳定性和成本。
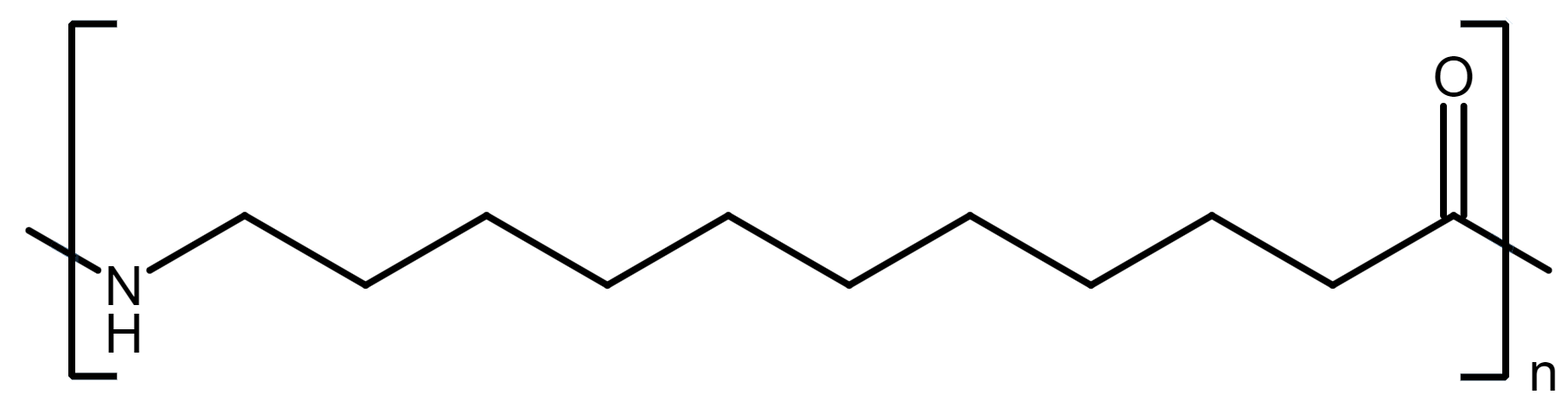
尼龙11是一种基于生物的长链聚酰胺,是通过11-氨基甲酸(来自蓖麻油)的自键合作产生的。它的长甲基段使它的极性远不及短链尼龙(例如PA6和PA66),因此它吸收的水分很少(在环境湿度下约0.2–0.3%),保持尺寸稳定,并保持潮湿环境中的电气。从机械上讲,它是坚硬且非常延展的(通常在200-300%的伸长率),即使在低温下也保持影响和疲劳性,因此实际上,它的行为更像是一种灵活的工程塑料,而不是坚固的塑料。
该长链结构的翻转侧是较低的拉伸强度/刚度和较低的耐热点(熔点〜185–190°C;适量的HDT),因此PA11通常不是通常指定PA66或PA66或PA4/6的高热,重载结构部件的理想选择。 PA11非常适合流体接触和室外服务:柔性燃料和气动制动线,软管/快速连接,有线夹克,密封件以及医疗或工业管。当需要坚硬的耐影零件时,它也是SLS 3D打印的主食粉末。与PA12相比,PA11提供了更高的熔点,通常会更好地紫外线/热空气老化,而PA12往往更柔软,更灵活。
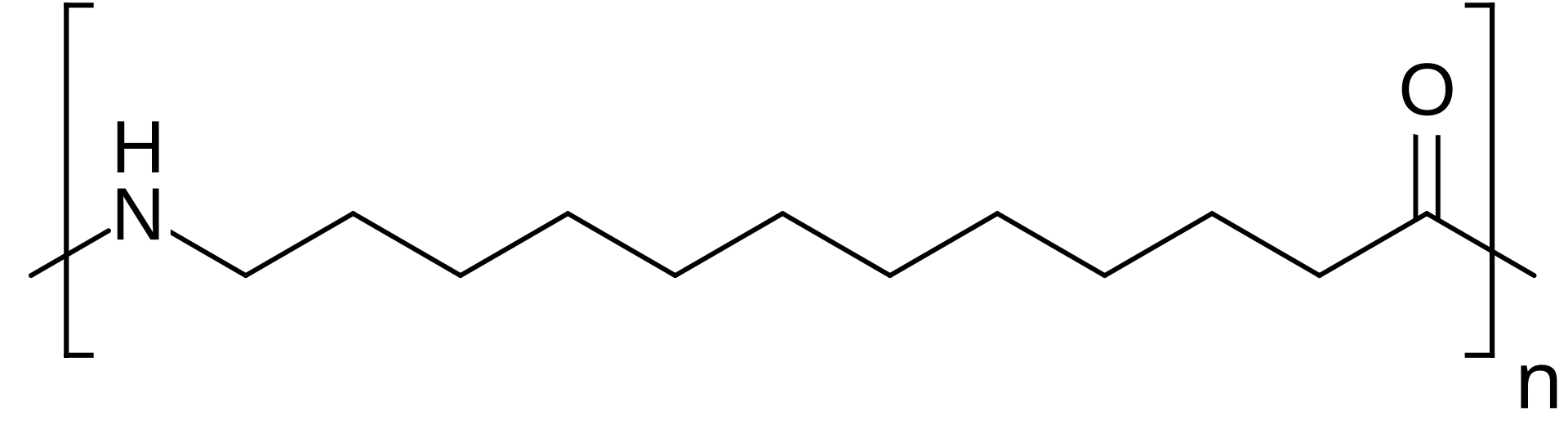
PA12是一种著名的“长链”尼龙,通常与诸如vestamid或grilamid之类的商品名称有关。尼龙12在化学上与尼龙11非常相似,通常被认为在许多用途中可以互换,但是存在细微的差异。尼龙12完全是石化的(通常来自丁二烯),而尼龙11是基于生物的可再生蓖麻油,这可能是可持续性的重要性。 PA11通常具有更高的熔点,在升高温度下的性能更好,并且通常显示出更好的紫外线抗性。另一方面,PA12的灵活性稍微更灵活(延伸〜300–400%对PA11的〜200–300%),并且模量略低,因此感觉更柔软。为了吸收水分和耐化学性,它们几乎是相同的 - 两者都很棒。
值得注意的是:PA12通常是最昂贵的尼龙之一(由于其基于生物的原料,PA11上的PA11或更高)。因此,当真正需要其独特的好处时,请使用PA12 - 您不会选择PA1的PA6就足够了,因为PA6便宜得多。总而言之,PA12在尼龙家族中提供了一些最佳的尺寸稳定性和耐化学性,即使在冰冻条件下也保持延展性,使其非常适合软管,密封,快速连接,有线夹克以及其他在湿,寒冷,寒冷或化学侵略性环境中都不会失败的部分。但是,它不像PA6或PA66那样强或耐热,因此它是专家,而不是通用的替代品。
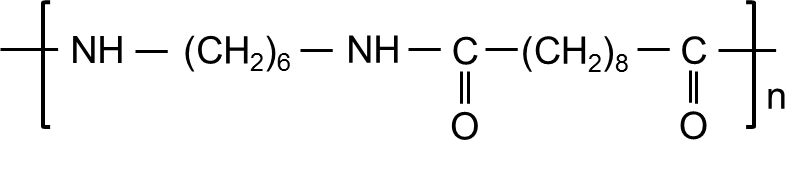
尼龙6/10(PA610)是早期的“低摩斯”尼龙之一,旨在解决PA66的湿度问题。每单位长度的酰胺组较少,它的极性较小,可吸收大约一半(或更少)PA6的水分,从而提供更好的尺寸稳定性。它还像其他长链尼龙一样显示出良好的伸长率,并保留在寒冷中的韧性,使其适用于室外或低温部分。与PA6/PA66相比,PA610的拉伸强度和刚度略低。总体而言,将PA610视为一种尼龙,可以进行一些强度和刚性,以提高水分稳定性和柔韧性。
它的熔点(〜220–225°C)和中等收缩使成型/挤出条件接近PA6。从化学上讲,PA610非常好:它可以抵抗大多数油和溶剂,并且在存在诸如氯化锌(可以积极攻击PA66)的盐的存在下对环境应力开裂具有明显抵抗力。由于其含量的一部分(脂肪酸)来自可再生能源,因此有时以更可持续的尼龙选择销售。经典用途包括刷毛和细丝(例如,牙刷和工业刷刷毛 - 历史上的杜邦“ Tynex”等级),单丝(钓线,杂草制成线)。在模制零件中,PA610用于电绝缘体/连接器,精密组件,拉链元件以及一些汽车燃油系统组件(尽管PA12和PA11占据了连续燃油管线)。与PA12相比,PA610更便宜且更强大,因此它可以替代少少的角色。简而言之,PA610作为中级尼龙填充了一个利基市场,以达到PA66的一些峰值强度,以获得PA12的大部分水分稳定性,通常以合理的成本;对于半湿环境或必须将特性保持寒冷的零件特别方便。
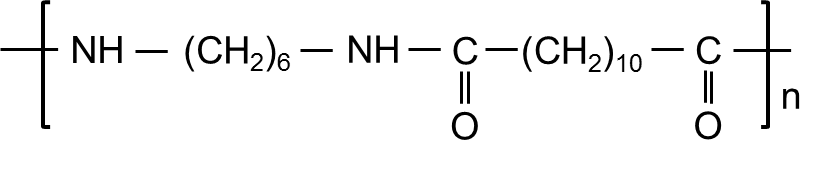
PA612(有时称为“ 612尼龙”)与PA610非常相似:与PA6/PA66相比,两者的水分吸收率低,尺寸稳定性差得多,在户外和低温下保持坚固,并且在215–218°C附近具有熔点,因此成型/挤出条件接近PA6。两者都非常适合流体处理连接器,精密电连接器和湿度暴露的零件,必须保持紧密的尺寸。
PA612的平衡水分吸收较低,其燃料/水蒸气渗透率较低,并且其湿状态的漂移较小,但通常成本更高。根据经验,请选择长期尺寸和电稳定性至关重要的湿环境中的PA612;当极度低温韧性或抵抗力在氯化锌 - 氯化物环境中的压力裂纹更重要,而成本敏感性更高时,请选择PA610。

每个尼龙等级 - 从尼龙6和6,6到短链脂肪族尼龙4,6和长链尼龙6,10、6,12、11和12-取得了独特的特性平衡。尼龙6和6,6是具有高强度和刚度的通用工作试义,适用于许多承重零件,但对水分敏感。尼龙4,6增加了耐热性,并保留了高温,高应力用途的高强度,尽管水分吸收和成本较高。转向更长的链条,尼龙6,10和6,12减少了水分的吸收,并以一点点强度为代价改善了韧性 - 对于需要在潮湿或冷设置中需要稳定性的零件。最后,尼龙11和12提供了最佳的水分和化学弹性和出色的韧性,使它们可以选择流体接触,户外和灵活的应用,尽管它们的较低熔点和更高的价格将它们限制在利基市场上,但关键的角色也关键了。
准备建造了吗? Chiggo专门研究CNC加工,3D打印和尼龙零件的注射成型。我们可以帮助您选择合适的成绩,优化设计以进行水分/收缩/扭曲,并从快速原型传递到生产。上传您的CAD快速的DFM审查和报价。

铸铁和钢都是主要由铁原子(在元素周期表中标记为 Fe)组成的黑色金属。元素铁在地球上含量丰富,但通常以氧化形式存在,需要经过深加工(称为熔炼)才能提取。

钣金弯曲是钣金制造中最常用的成形技术之一。根据具体应用,有时称为折弯、翻边、模具弯曲、折叠或磨边。该过程涉及施加力使材料变形为有角形状。

从日常的家居用品到高性能的工业组件,塑料制造有助于塑造我们周围的世界。这些组件的各种形状和功能是使用一系列制造工艺制成的,包括注入成型,塑料挤出,3D打印等。这些方法在塑料部分生产中有什么区别,哪种方法(或组合)最适合您的项目?预算,零件设计,塑料材料和生产量只是选择塑料制造方法时出现的一些因素。本文介绍了11种常见的塑料制造方法,解释了它们的工作方式,益处,局限性和典型应用。

عربي
عربي
中国大陆
简体中文
United Kingdom
English
France
Français
Deutschland
Deutsch
नहीं
नहीं
日本
日本語
Português
Português
España
Español